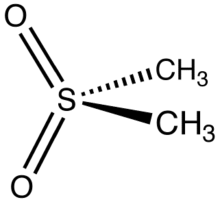
Methylsulfonylmethane
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Methanesulfonyl)methane | |
| Other names
methyl sulfone
methylsulfonylmethane sulfonylbismethane DMSO2 | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | MSM |
| 1737717 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.605 |
| EC Number |
|
| 130437 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H6O2S | |
| Molar mass | 94.13 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.45 g/cm3 |
| Melting point | 109 °C (228 °F; 382 K) |
| Boiling point | 248[1] °C (478 °F; 521 K) |
| Acidity (pKa) | 31 |
| Hazards[3] | |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P264, P280, P305+P351+P338, P337+P313 | |
| NFPA 704 (fire diamond) | |
| Flash point | 143 °C (289 °F; 416 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
5g/kg (oral, rat) [2] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
DMSO dimethyl sulfide dimethyl sulfate sulfolane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethyl sulfone (DMSO2) is an organosulfur compound with the formula (CH3)2SO2. It is also known by several other names including methyl sulfone and (especially in alternative medicine) methylsulfonylmethane (MSM).[4] This colorless solid features the sulfonyl functional group and is the simplest of the sulfones. It is relatively inert chemically and is able to resist decomposition at elevated temperatures. It occurs naturally in some primitive plants, is present in small amounts in many foods and beverages, and is marketed (under the MSM name) as a dietary supplement. It is sometimes used as a cutting agent for illicitly manufactured methamphetamine.[5] It is also commonly found in the atmosphere above marine areas, where it is used as a carbon source by the airborne bacteria Afipia.[6] Oxidation of dimethyl sulfoxide produces the sulfone, both under laboratory conditions and metabolically.[7]
Use as a solvent
[edit]Because of its polarity and thermal stability, molten DMSO2 has been used industrially as a high-temperature solvent. For example, displacement of aryl chlorides by potassium fluoride has been conducted in the liquid.[8]
With a pKa of 31, the sulfone can be deprotonated with sodium amide. The conjugate base has been used as a nucleophile.
Pharmacology and toxicity
[edit]Nuclear magnetic resonance (NMR) studies have demonstrated that oral doses of MSM are absorbed into the blood and cross the blood/brain barrier.[9][10] An NMR study has also found detectable levels of MSM normally present in the blood and cerebrospinal fluid, suggesting that it derives from dietary sources, intestinal bacterial metabolism, and the body's endogenous methanethiol metabolism.[11]
Medical and dietary use
[edit]No medical uses for MSM have been approved. A variety of unproven health benefits have been claimed by promoters such as Stanley W. Jacob.[12][13]
Some promoters suggest that most people do not consume enough sulfur. There is no Dietary Reference Intake (DRI) or Daily Value established for sulfur, but it is present in significant amounts in foods such as cruciferous vegetables, garlic, onions, asafoetida, legumes, nuts, seeds, plant milk, animal milk and eggs (both whites and yolks).[14] The claims for the need for sulfur supplementation originate with Robert Herschler, a biochemist who filed a patent for using MSM as a dietary supplement in 1982.[15]
Moreover, in cases involving topical therapeutics, the role of MSM as an active agent, per se, versus its having a role in promoting skin permeation (in manner, akin to its solvent relative DMSO) must be characterized/controlled.[16]
The biochemical effects of supplemental methylsulfonylmethane are poorly understood. Some researchers have suggested that MSM has anti-inflammatory effects.[17]
The spectrum of biological effects of dimethyl sulfoxide (DMSO) and MSM differ, but those of DMSO may be mediated, at least in part, by MSM.[18]
Generally recognized as safe
[edit]In July 2007 a manufacturer of MSM submitted a notification to the U.S. FDA claiming generally recognized as safe (GRAS) status. GRAS status is for safety, and has no evaluation of efficacy. The FDA responded in February 2008 with a letter of non-objection, functionally designating OptiMSM, the branded form of MSM, as GRAS. The designation allows MSM to be added to processed foods.[19]
Evidence from clinical trials
[edit]Small studies of MSM have suggested some benefit for treatment of oxidative stress and osteoarthritis, but evidence for other uses is lacking.[20]
Safety
[edit]The LD50 of MSM is greater than 17.5 grams per kilogram of body weight.[21] Extensive research in animal models indicates MSM has a very low toxicity when administered both orally and topically.[22][23][24] MSM is considered 'Possibly Safe' at therapeutic doses.[20][25]
Osteoarthritis
[edit]Methylsulfonylmethane may or may not be useful for osteoarthritis (OA) knee pain relief. Methodological issues with the clinical trials prevent firm conclusions, and the review authors stated: "No definitive conclusion can currently be drawn" and there is "no definitive evidence that MSM is superior to placebo in the treatment of mild to moderate osteoarthritis of the knee."[26]
Skin, hair, nails
[edit]The first scientific investigation of MSM for skin health was published in 2015. The RCT used a 3g per day dose and included only 20 women. Results showed significant improvements in the number and severity of facial wrinkles, firmness, tone and texture.[27] Another study evaluated doses of 1g and 3g and showed improvements in wrinkles, firmness, and hydration at both dose levels in 20 persons.[28] The same author published a study on MSM for hair and nails from the same clinical trial. The results showed improvements in hair shine, volume, and appearance, and nail shine and appearance.[29] An Italian study evaluated the effects of a nutraceutical composed of MSM, hyaluronic acid, and L-carnosine. The results from RCT showed broad improvements in facial skin hydration and elasticity, as well as decreased sebaceous secretions.[30]
Oxidative stress and inflammation
[edit]Multiple human and animal trials indicate MSM may reduce oxidative stress and inflammation. In one small human trial, MSM has been shown to protect muscles from damage by reducing the amount of oxidative stress damage incurred through exercise.[31][32] In a second small trial the total antioxidant capacity was significantly increased after taking MSM.[33] Studies in animals indicate a hepatoprotective effect of MSM against several toxins including acetaminophen, paraquat, and carbon tetrachloride.[34][35][36][37] Animal models of experimental colitis and pulmonary hypertension indicate a protective effect as well.[38][39]
Allergies and immunity
[edit]Two studies have evaluated the effects of MSM on allergic rhinitis. A 2004 multi-centered, open-label clinical trial found that MSM reduced both upper and lower respiratory symptoms associated with seasonal allergic rhinitis (SAR), and increased energy levels. It found no significant changes in IgE levels, although the duration of the study was not likely long enough to see changes.[40] An RCT evaluated three doses of MSM and found that a 3g daily dose was most effective compared to 1g or 6g per day. Daily use at 3g decreased allergy-associated symptoms, including itchy eyes, itchy nose, watery eyes, rhinorrhea, sneezing, and nasal obstruction. The 3g dose also improved peak nasal inspiratory flow (PNIF) indicating improved breathing. The study also evaluated an acute 12g dose and found significant improvements in all symptoms except itching eyes and sneezing, but not for PNIF.[41]
MSM has been shown to improve immune function markers. RCT found that in blood samples taken after bouts of exhaustive exercise, there was a reduced response to an infectious stimulus in the placebo group, but the MSM group maintained a robust response, indicating that MSM protected against stress-induced immunosuppression. The authors postulate that MSM’s anti-inflammatory properties reduce the overstimulation of inflammatory cells during exercise, thus conserving their ability to respond to infections threats.[42] This is supported by in vitro research showing MSM inhibits over-activation of white blood cells and has an anti-apoptotic effect.[43][44]
Notes
[edit]- ^ Gaylord Chemical Company, LLC
- ^ "Dimethyl sulfone".
- ^ "Dimethyl sulfone". pubchem.ncbi.nlm.nih.gov.
- ^ "Various Names for MSM" (PDF). Archived from the original (PDF) on July 11, 2011. Retrieved June 8, 2009.
- ^ "Information Bulletin: Crystal Methamphetamine". www.justice.gov. Retrieved 20 January 2018.
- ^ DeLeon-Rodriguez N, Lathem TL, Rodriguez-R LM, Barazesh JM, Anderson BE, Beyersdorf AJ, Ziemba LD, Bergin M, Nenes A, Konstantinidis KT (February 2013). "Microbiome of the upper troposphere: species composition and prevalence, effects of tropical storms, and atmospheric implications". Proceedings of the National Academy of Sciences of the United States of America. 110 (7): 2575–80. Bibcode:2013PNAS..110.2575D. doi:10.1073/pnas.1212089110. PMC 3574924. PMID 23359712.
This group [Afipia] is commonly found in aquatic environments and is known to use dimethyl sulfone (DMSO2) as a sole carbon source. DMSO2 represents an intermediate of the oxidation of dimethyl sulfide (DMS), which is commonly found in the marine atmosphere
(page 5 of 6, quote slightly edited). - ^ He X, Slupsky CM (December 2014). "Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism". Journal of Proteome Research. 13 (12): 5281–92. doi:10.1021/pr500629t. PMID 25245235.
- ^ Hareau G, Kocienski P (2001). "Dimethyl Sulfone". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd371. ISBN 978-0471936237.
- ^ Rose SE, Chalk JB, Galloway GJ, Doddrell DM (January 2000). "Detection of dimethyl sulfone in the human brain by in vivo proton magnetic resonance spectroscopy". Magnetic Resonance Imaging. 18 (1): 95–8. doi:10.1016/S0730-725X(99)00110-1. PMID 10642107.
- ^ Lin A, Nguy CH, Shic F, Ross BD (September 2001). "Accumulation of methylsulfonylmethane in the human brain: identification by multinuclear magnetic resonance spectroscopy". Toxicology Letters. 123 (2–3): 169–77. doi:10.1016/S0378-4274(01)00396-4. PMID 11641045.
- ^ Engelke UF, Tangerman A, Willemsen MA, Moskau D, Loss S, Mudd SH, Wevers RA (August 2005). "Dimethyl sulfone in human cerebrospinal fluid and blood plasma confirmed by one-dimensional (1)H and two-dimensional (1)H-(13)C NMR". NMR in Biomedicine. 18 (5): 331–6. doi:10.1002/nbm.966. PMID 15996001. S2CID 34324509.
- ^ Jacob S (2003). MSM the Definitive Guide: Nutritional Breakthrough for Arthritis, Allergies and More. Freedom Press. ISBN 978-1-893910-22-5.[page needed]
- ^ Jacob S, Lawrence RM, Zucker M (1999). The Miracle of MSM: The Natural Solution for Pain. New York: Penguin-Putnam.
- ^ Lang KL (17 June 2001). "Methylsulfonylmethane (MSM)". Quackwatch. Retrieved 2011-03-12.
- ^ US granted 4514421, Herschler RJ, "Dietary and pharmaceutical uses of methylsulfonylmethane and compositions comprising it", issued 30 April 1985
- ^ Shanmugam S, Baskaran R, Nagayya-Sriraman S, Yong CS, Choi HG, Woo JS, Yoo BK (31 July 2009). "The Effect of Methylsulfonylmethane on Hair Growth Promotion of Magnesium Ascorbyl Phosphate for the Treatment of Alopecia". Biomolecules and Therapeutics. 17 (3): 241–248. doi:10.4062/biomolther.2009.17.3.241.
- ^ Morton JI, Siegel BV (November 1986). "Effects of oral dimethyl sulfoxide and dimethyl sulfone on murine autoimmune lymphoproliferative disease". Proceedings of the Society for Experimental Biology and Medicine. 183 (2): 227–30. doi:10.3181/00379727-183-42409. PMID 3489943. S2CID 23242700.
- ^ Kocsis JJ, Harkaway S, Snyder R (January 1975). "Biological effects of the metabolites of dimethyl sulfoxide". Annals of the New York Academy of Sciences. 243 (1): 104–9. Bibcode:1975NYASA.243..104K. doi:10.1111/j.1749-6632.1975.tb25349.x. PMID 1055534. S2CID 45625081.
- ^ "Agency Response Letter GRAS Notice No. GRN 000229". fda.gov. 2008.
- ^ a b "MSM Monograph". Natural Medicines Comprehensive Database. Therapeutic Research Faculty. Retrieved 10 Jan 2024.(subscription required)
- ^ Horváth K, Noker PE, Somfai-Relle S, Glávits R, Financsek I, Schauss AG (October 2002). "Toxicity of methylsulfonylmethane in rats". Food and Chemical Toxicology. 40 (10): 1459–62. doi:10.1016/S0278-6915(02)00086-8. PMID 12387309.
- ^ Debbi EM, Agar G, Fichman G, Ziv YB, Kardosh R, Halperin N, Elbaz A, Beer Y, Debi R (June 2011). "Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study". BMC Complementary and Alternative Medicine. 11: 50. doi:10.1186/1472-6882-11-50. PMC 3141601. PMID 21708034.
- ^ Pagonis TA (2014). "The Effect of Methylsulfonylmethane on Osteoarthritic Large Joints and Mobility". International Journal of Orthopaedics. 1 (1): 19–24. doi:10.6051/j.issn.2311-5106.2014.01.7 (inactive 1 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Takiyama K, Konishi F, Nakashima Y, Mumamoto C (2010). "Single and 13-week Repeated Oral Dose Toxicity Study of Methylsulfonylmethane in Mice". Oyo Yakuri Pharmacometrics. 79: 23–30.
- ^ Bauer BA (6 June 2014). "MSM for arthritis pain: Is it safe?". Expert Answers. Mayo Clinic. Retrieved 14 July 2015.
- ^ Brien S, Prescott P, Bashir N, Lewith H, Lewith G (November 2008). "Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis". Osteoarthritis and Cartilage. 16 (11): 1277–88. doi:10.1016/j.joca.2008.03.002. PMID 18417375.
- ^ Anthonavage, Michael; Benjamin, Rod; Withee, Eric (2015). "Effects of oral supplementation with methylsulfonylmethane on skin health and wrinkle reduction". Natural Medicine Journal. 7 (11): 1–21.
- ^ Muizzuddin, Neelam; Benjamin, Rodney (2020-02-21). "Beauty from within: Oral administration of a sulfur-containing supplement methylsulfonylmethane improves signs of skin ageing". International Journal for Vitamin and Nutrition Research. 92 (3–4): 182–191. doi:10.1024/0300-9831/a000643. ISSN 0300-9831. PMID 32083522. S2CID 211232166.
- ^ Muizzuddin, Neelam; Benjamin, Rodney (2019). "Beneficial Effects of a Sulfur-Containing Supplement on Hair and Nail Condition". Natural Medicine Journal. 11 (11): 1–8.
- ^ Guaitolini, Ennio; Cavezzi, Attilio; Cocchi, Stefania; Colucci, Roberto; Urso, Simone Ugo; Quinzi, Valentina (2019). "Randomized, Placebo-controlled Study of a Nutraceutical Based on Hyaluronic Acid, L-carnosine, and Methylsulfonylmethane in Facial Skin Aesthetics and Well-being". The Journal of Clinical and Aesthetic Dermatology. 12 (4): 40–45. ISSN 1941-2789. PMC 6508480. PMID 31119010.
- ^ Barmaki S, Bohlooli S, Khoshkhahesh F, Nakhostin-Roohi B (April 2012). "Effect of methylsulfonylmethane supplementation on exercise - Induced muscle damage and total antioxidant capacity". The Journal of Sports Medicine and Physical Fitness. 52 (2): 170–4. PMID 22525653.
- ^ Nakhostin-Roohi B, Barmaki S, Khoshkhahesh F, Bohlooli S (October 2011). "Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men" (PDF). The Journal of Pharmacy and Pharmacology. 63 (10): 1290–4. doi:10.1111/j.2042-7158.2011.01314.x. PMID 21899544. S2CID 25474694.
- ^ Nakhostin-Roohi B, Niknam Z, Vaezi N, Mohammadi S, Bohlooli S (2013). "Effect of single dose administration of methylsulfonylmethane on oxidative stress following acute exhaustive exercise". Iranian Journal of Pharmaceutical Research. 12 (4): 845–53. PMC 3920715. PMID 24523764.
- ^ Bohlooli S, Mohammadi S, Amirshahrokhi K, Mirzanejad-Asl H, Yosefi M, Mohammadi-Nei A, Chinifroush MM (August 2013). "Effect of Methylsulfonylmethane Pretreatment on Aceta-minophen Induced Hepatotoxicity in Rats". Iranian Journal of Basic Medical Sciences. 16 (8): 896–900. PMC 3786100. PMID 24106592.
- ^ Amirshahrokhi K, Bohlooli S (October 2013). "Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice". Inflammation. 36 (5): 1111–21. doi:10.1007/s10753-013-9645-8. PMID 23595869. S2CID 2209805.
- ^ Kamel R, El Morsy EM (September 2013). "Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats". Archives of Pharmacal Research. 36 (9): 1140–8. doi:10.1007/s12272-013-0110-x. PMID 23591777. S2CID 27990171.
- ^ Disilvestro RA, Disilvestro DJ, Disilvestro DJ (2008). "Methylsulfonylmethane (MSM) Intake in Mice Produces Elevated Liver Glutathione and Partially Protects Against Carbon Tetrachloride-Induced Liver Injury". FASEB J. 22 (1): 445–8. doi:10.1096/fasebj.22.1_supplement.445.8. S2CID 84049802.
- ^ Amirshahrokhi K, Bohlooli S, Chinifroush MM (June 2011). "The effect of methylsulfonylmethane on the experimental colitis in the rat". Toxicology and Applied Pharmacology. 253 (3): 197–202. Bibcode:2011ToxAP.253..197A. doi:10.1016/j.taap.2011.03.017. PMID 21463646.
- ^ Mohammadi S, Najafi M, Hamzeiy H, Maleki-Dizaji N, Pezeshkian M, Sadeghi-Bazargani H, Darabi M, Mostafalou S, Bohlooli S, Garjani A (2012). "Protective effects of methylsulfonylmethane on hemodynamics and oxidative stress in monocrotaline-induced pulmonary hypertensive rats". Advances in Pharmacological Sciences. 2012: 1–6. doi:10.1155/2012/507278. PMC 3478703. PMID 23118745.
- ^ Barrager, Eleanor; Veltmann, Joseph R.; Schauss, Alexander G.; Schiller, Rebecca N. (2002). "A Multicentered, Open-Label Trial on the Safety and Efficacy of Methylsulfonylmethane in the Treatment of Seasonal Allergic Rhinitis". The Journal of Alternative and Complementary Medicine. 8 (2): 167–173. doi:10.1089/107555302317371451. ISSN 1075-5535. PMID 12006124.
- ^ Hewlings, Susan; Kalman, Douglas S (2018-11-29). "Evaluating the Impacts of Methylsulfonylmethane on Allergic Rhinitis After a Standard Allergen Challenge: Randomized Double-Blind Exploratory Study". JMIR Research Protocols. 7 (11): e11139. doi:10.2196/11139. ISSN 1929-0748. PMC 6293242. PMID 30497995.
- ^ van der Merwe, Mariè; Bloomer, Richard J. (2016). "The Influence of Methylsulfonylmethane on Inflammation-Associated Cytokine Release before and following Strenuous Exercise". Journal of Sports Medicine. 2016: 7498359. doi:10.1155/2016/7498359. ISSN 2356-7651. PMC 5097813. PMID 27844051.
- ^ Kim, Yoon Hee; Kim, Dae Hwan; Lim, Hwan; Baek, Doo-Yeon; Shin, Hyun-Kyung; Kim, Jin-Kyung (2009). "The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages". Biological & Pharmaceutical Bulletin. 32 (4): 651–656. doi:10.1248/bpb.32.651. ISSN 0918-6158. PMID 19336900.
- ^ Karabay, Arzu Z.; Aktan, Fugen; Sunguroğlu, Asuman; Buyukbingol, Zeliha (2014). "Methylsulfonylmethane modulates apoptosis of LPS/IFN-γ-activated RAW 264.7 macrophage-like cells by targeting p53, Bax, Bcl-2, cytochrome c and PARP proteins". Immunopharmacology and Immunotoxicology. 36 (6): 379–389. doi:10.3109/08923973.2014.956752. ISSN 0892-3973. PMID 25211405. S2CID 29105910.

